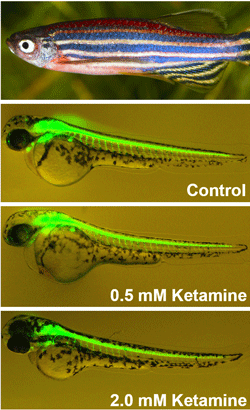
Lampire Biological Laboratories, Inc., is pleased to announce that a publication entitled Inter-laboratory assessment of a harmonized zebrafish developmental toxicology assay – progress report on phase I was recently awarded the “Best Manuscript in Reproductive Toxicology, 2012” by the Officers of the European Teratology Society. This publication came as a result of Lampire’s participation in a multi-pharma consortium whose goal is to develop a harmonized zebrafish developmental toxicity assay, and included Senior Toxicologist Dr. Jedd Hillegass as one of its authors. In recognition of this achievement, the European Teratology Society invited a fellow member of the consortium to present this work at the Elsevier Reproductive Toxicology Publication Award Lecture during the 2013 meeting in Stresa, Italy.
Please feel free to contact Lampire to see how our zebrafish embryo culture (ZEC) screening service can assist you in determining the teratogenic liability of your compound of interest. If you would like more information, please contact our Senior Toxicologist, Dr. Jedd Hillegass (jhillegass@lampire.com; 215-766-4901). This assay, along with our rat whole embryo culture (rWEC) assay, are just two examples of the continuing Lampire initiative to provide cost effective in vitro teratogenicity screening assays that conform to the principle of reducing, refining, and replacing animals used in research.